Published online Mar 27, 2015. doi: 10.4254/wjh.v7.i3.607
Peer-review started: August 28, 2014
First decision: November 3, 2014
Revised: December 11, 2014
Accepted: December 29, 2014
Article in press: December 29, 2014
Published online: March 27, 2015
Liver fibrosis is a common histological change of chronic liver injury and it is closely related with portal hypertension which is hemodynamic complication of chronic liver disease. Currently, liver fibrosis has been known as a reversible dynamic process in previous literatures. Although liver biopsy is a gold standard for assessing the stage of liver fibrosis, it may not completely represent the stage of liver fibrosis because of sampling error or semi-quantative measurement. Recent evidences suggested that histologic, clinical, hemodynamic, and biologic features are closely associated in patients with chronic liver disease. Hepatic venous pressure gradient (HVPG) measurement has been known as a modality to evaluate the portal pressure. The HVPG measurement has been used clinically for fibrosis diagnosis, risk stratification, preoperative screening for liver resection, monitoring the efficacy of medical treatments, and assessing the prognosis of liver fibrosis. Therefore, the HVPG measurement can be used to monitor areas the chronic liver disease but also other important areas of chronic liver disease.
Core tip: Hepatic venous pressure gradient (HVPG) measurement has been used in the clinical fields such as diagnosis of fibrosis, risk stratification, preoperative screening for liver resection, monitoring of the efficacy of medical treatment, and assessing the prognosis of liver fibrosis. HVPG measurement, along with monitoring stage the liver fibrosis, will play important roles in the field of chronic liver disease.
- Citation: Suk KT, Kim DJ. Staging of liver fibrosis or cirrhosis: The role of hepatic venous pressure gradient measurement. World J Hepatol 2015; 7(3): 607-615
- URL: https://www.wjgnet.com/1948-5182/full/v7/i3/607.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i3.607
Liver fibrosis is one of the leading causes of mortality because it changes the architecture of certain organs and disrupts normal function[1,2]. Liver fibrosis is a histological consequence of the wound-healing process resulting from chronic liver diseases such as viral hepatitis, alcoholic liver disease, non-alcoholic fatty liver disease, and other liver disorders. Deposition of excess extracellular matrix (ECM) that is rich in fibril-forming collagens is a typical finding of liver fibrosis[3]. The excess deposition of the ECM changes the normal architecture of the liver resulting in pathophysiologic damage to the organ.
Liver cirrhosis (LC) is defined as an advanced stage of liver fibrosis with distortion of the hepatic vasculature and architecture. Histologically, regenerative nodules with fibrous tissues form in response to chronic injury and lead to LC[4,5]. Consequently, disruption of the liver architecture due to liver fibrosis and/or cirrhosis causes hemodynamic instability and portal hypertension. The development of portal hypertension is a hallmark of LC.
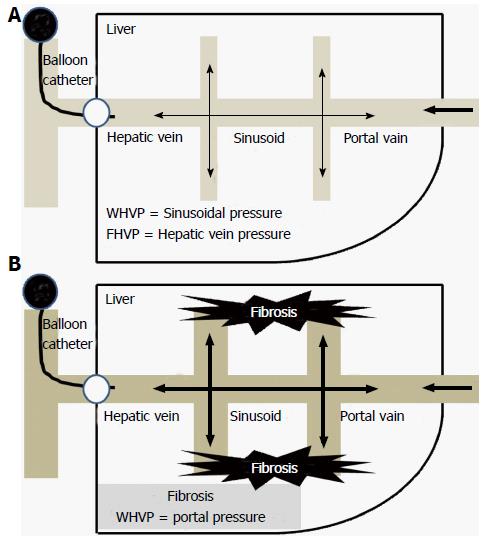
Portal hypertension is a clinical syndrome defined by an increase in the hepatic venous pressure gradient (HVPG) above 5 mmHg due to increased hepatic resistance[6]. Portal hypertension occurs in patients with fibrosis in the sinusoid of the liver, and portal hypertension is one of the causes of several severe complications of LC (variceal bleeding, ascites, peritonitis, or hepatic encephalopathy) that are associated with its mortality[7,8].
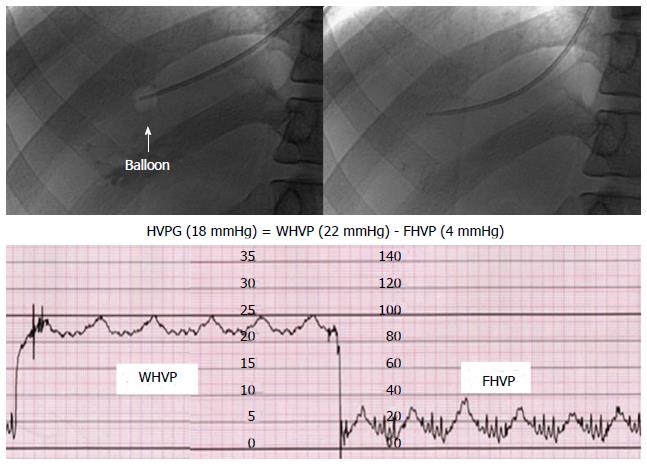
The concept of wedged hepatic venous pressure (WHVP) was described by Myers and Taylor. WHVP can be measured by occlusion of hepatic vein using catheterization[9]. For many years, a safe, simple, reproducible, and less invasive method has been used to measure the HVPG. HVPG means the difference between the portal vein pressure and the hepatic vein pressure. Measuring both the free hepatic venous pressure (FHVP) and the WHVP has been the standard method for estimating HVPG[10-12].
It has been proposed that serial HVPG measurements can estimate the stage of fibrosis or cirrhosis regardless of the etiology[13,14]. In addition, the HVPG measurement has been used clinically for fibrosis diagnosis, risk stratification, preoperative screening for liver resection, monitoring the efficacy of medical treatments, and assessing the prognosis of liver fibrosis. Therefore, the HVPG measurement can be used to monitor areas the chronic liver disease but also other important areas of chronic liver disease[15]. This review presents the role of the HVPG measurement in staging liver fibrosis and LC.
Because liver fibrosis is positively related to prognosis, accurate staging of liver fibrosis gives important clinical implications in the management of chronic liver disease[16]. As such, there is a strong demand for reliable liver fibrosis biomarkers that provide insight into the disease etiology, diagnosis, therapy, and prognosis[17]. Currently, diagnostic modalities range from blood biomarkers to genomics as well as to even more advanced techniques such as elastography, or magnetic resonance imaging[17].
The mechanism of liver fibrosis is thought to be associated with the hepatic damage of various etiologic factors followed by the activation of hepatic stellate cells (HSC) within the liver that develop into myofibroblasts[18]. HSCs are resident peri-sinusoidal cells in the subendothelial space between hepatocytes and sinusoidal endothelial cells[19]. The main cells affected by liver fibrosis are the HSCs and fibroblasts, which are activated by soluble mediators produced by activated Kupffer cells or inflammatory cells in the course of chronic liver disease[20,21].
HSCs activation consists of 3 phases (initiation, perpetuation, and final resolution phase when liver injury resolves)[19]. Initiation is the first phase occurred during HSC activation resulting from paracrine activation by all other cells such as sinusoid endothelium, hepatocytes, cholangiocyte, and platelets. Hepatocyte apoptosis caused by injury also promotes HSCs initiation through a process mediated in part by Fas, tumor necrosis factor-related apoptosis-inducing ligand, endothelial cells, platelets, and Kupffer cells[19,22,23].
The perpetuation phase of HSCs results from the chronic stimulation that signals for cell maintenance of the activated form and induces liver fibrosis[24]. The perpetuation of HSC activation causes changes in cell behavior, including proliferation, chemotaxis, fibrogenesis, contractility, matrix degradation, and retinoid loss. The perpetuation phase involves autocrine and paracrine loops. The effect of these changes leads to the accumulation of the ECM in the liver. Activated HSCs become directly fibrogenic by increasing the synthesis and deposition of ECM proteins[24]. The contractility of HSCs may be one of the main causes of elevated portal resistance during liver fibrosis. The collagen bands in LC contain a large numbers of activated HSCs[25]. Liver fibrosis is caused by an unbalance between matrix production and degradation. A main component of ECM remodeling is the family of matrix-metalloproteinases[26]. In addition, the tissue inhibitors of metalloproteinases and the uroplasminogen activator receptor or its inhibitor as well as other components of the plasmin system are closely related to ECM degradation[26-28]. In the case of liver fibrosis resolution, HSCs are either driven to apoptosis or prompted to revert to a quiescent HSC[5,24].
The change from initial liver fibrosis to LC involves the inflammation and activation of HSCs with subsequent fibrogenesis, angiogenesis, and parenchymal disruption caused by vascular occlusion[29]. Histologically, LC is characterized by a vascularized fibrosis septum that allows for communication between portal tracts and to the central veins, resulting in liver nodules that are devoid of a central vein and surrounded by a fibrotic band[30]. This vascular distortion leads to shunting of the blood supply between the portal vein and artery and disrupts the exchange between hepatic sinusoids and the liver parenchyma. The hepatic sinusoids are lined by endothelium that is located on a sheet of permeable connective tissue in the space of Disse, in which HSCs and other cells also rest[31]. Hepatocytes, perform most of the known liver functions and line the other side of the space of Disse. In LC, the space of Disse becomes occupied with fibrous tissue, and the endothelium loses its functions, a process known as sinusoidal capillarization[32].
Increased resistance to portal blood flow is the main cause of increased portal pressure in LC. Portal hypertension results from the structural distortion that is associated with advanced fibrosis and LC (Figure 1). LC and the resultant vascular distortion were previously regarded as irreversible. However, recent reliable data suggest that LC regression or even reversal is possible[33].
Liver biopsy is currently the gold standard for assessing liver fibrosis and has been used for the diagnosis of fibrosis, risk stratification, prognosis evaluation, and differential diagnoses. In the 1960s, the introduction of the liver biopsy brought about substantial change in the field of liver disease[34]. Currently, several semi-quantitative scoring systems are available for the diagnosis of liver fibrosis (METAVIR, Knodell, and the Ishak score) (Table 1)[35]. Typically, liver fibrosis is scored in stages and necro-inflammation is evaluated by grade. Liver fibrosis is histologically staged by assessing the amount of fibrosis and level of architectural disorganization. Until now, these semi-quantitative scoring systems have been used in many clinical trials and for the evaluation of chronic liver disease. Though the current scoring systems apply a common principle to assess the status of chronic liver disease, none of them specifically describes the relation between these scoring systems and the level of liver fibrosis[36]. Because LC is defined as a diffuse process in which the normal lobules are replaced by architecturally abnormal nodules separated by fibrous tissue, the semi-quantitative nature of these histologic scoring systems do not fully represent the actual state of the liver nor do they include the histologic features of LC have been traditionally linked to clinical outcomes[37]. In addition, tissue obtained by liver biopsy is only a small portion of the entire liver (1/50000); therefore, sampling error may be inevitable[38].
| Score | |||||||
| METAVIR score | 0 | 1 | 1 | 2 | 3 | 4 | 4 |
| No fibrosis | Portal fibrosis without septa | Portal fibrosis without septa | Septal fibrosis (portal-portal) | Septal fibrosis (portal-central) | Cirrhosis | Cirrhosis | |
| Ishak score | 1 | 2 | 3 | 4 | 5 | 6 | |
| No fibrosis | Some portal tract fibrotic ± short fibrous septa | Most portal tract fibrotic ± short fibrous septa | Portal tract fibrotic with occasional portal to portal bridging | Portal tract fibrotic with marked portal to portal and portal to central bridging | Marked portal to portal and/or portal to central with occasional nodules | Cirrhosis | |
Taken together, liver histology may be the best-standardized method for evaluating the status of chronic liver disease. However, a comprehensive study of chronic liver disease requires data that combine all histological, hemodynamic, and clinical features as well as clinical endpoints, such as the onset of complications of cirrhosis related to LC and the incidence of death[33,39,40].
Hemodynamically, the sinusoidal connection dissipates the pressure backup from the wedged catheter. Consequently, WHVP is slightly lower than the directly measured portal pressure. In LC, the inter-sinusoidal communication becomes disrupted by the fibrosis septum and tissue, thus the reduction of WHVP becomes blocked. Therefore, the WHVP accurately represents the portal pressure (Figure 1)[41]. The use of a balloon catheter allows for the occlusion of a branch of the large hepatic vein at the lobar and sub-lobar levels. As a result, the hemodynamic stage of a large portion of the liver can be measured via the HVPG.
Three veins (antecubital, femoral, or right jugular vein) have been commonly used as route for catheter insertion for the HVPG measurement. A 6 or 7 French balloon catheter is placed in the hepatic vein through a guide track made in the vein to measure the FHVP and FHVP. The WHVP is measured by inflating the balloon. And then, the HVPG is calculated by subtracting the FHVP from the WHVP (Figure 2)[11,42].
The HVPG measurement has been a useful tool for the diagnosis, evaluation and assessment of the severity and prognosis of chronic liver disease and cirrhosis, including the risk assessment of the LC related complications[43]. Compensated LC is defined according to the presence of varices[44]. Patients with an HVPG ≤ 10 mmHg had a 90% possibility of maintaining compensated LC during a median follow-up of four years[45]. Ripoll et al[46] demonstrated that an HVPG > 10 mmHg increases the risk of clinical decompensation such as bleeding, ascites, hyperbilirubinemia, or encephalopathy. In other reports, patients with LC and an HVPG > 16 mmHg or > 20 mmHg showed a poor prognosis[47-49].
In patients with stage 1 compensated LC, the sensitivity and specificity of the HVPG in predicting stage 1 compensated LC were 78% and 81% at an HVPG of 6 mmHg, respectively[50]. Other reports have also suggested a significant correlation between the HVPG and fibrosis stage[51]. The area under receiver operating characteristic (AUROC) curve of HVPG for the diagnosis of advanced fibrosis was 0.906. The HVPG > 13 mmHg revealed a sensitivity of 79% and specificity of 89% in the prediction of advanced fibrosis[51]. In another study, the HVPG showed a good AUROC of 0.85 for the prediction of advanced fibrosis among patients with chronic viral hepatitis and a sensitivity and specificity of 80% and 77%, respectively, which were superior to that of other serologic biomarkers[52]. In addition, it has been demonstrated that the HVPG is associated with critical complications such as portal hypertension, HCC, and survival[53,54]. Repeated HVPG measurements might assess the progression of fibrosis to cirrhosis despite the lack of other etiologic factors[13,55].
Currently, liver stiffness measurements by transient elastography have been a promising and safe method used to monitor fibrosis progression and to predict portal hypertension in patients with chronic liver disease[56-58]. In patients who had undergone liver transplantation, the HVPG score was correlated with the liver stiffness measurement in the overall population[56]. The positive relation between liver stiffness and the HVPG score has been found in patients with LC, especially those with an HVPG < 10 mmHg[56]. The AUROC for predicting HVPG values of 10 mmHg and 12 mmHg is reported as 0.76 and 0.99 (cutoff value 13.6 kPa and 34.9 kPa), respectively[56,59]. In another study, HVPG scores of > 6 mmHg and > 10 mmHg were predicted by a cutoff value of 8.7 kPa and 21 kPa, respectively[60].
In patients with LC, the annual incidence rate of variceal bleeding is estimated to be 4%. However, this bleeding risk might be as high as 15% according to the size of the varices[61], and an HVPG > 10 mmHg is considered a good predictor of the development of varices[45]. In one study, an HVPG score of 11 mmHg had sensitivity and specificity for variceal hemorrhage of 92.4% and 27.7%, respectively[62].
In patients with LC, the probability of incidenct bleeding at 3 years after being diagnosed with LC was significantly higher in poor responders than in good responders to therapy with beta-blockers alone or beta-blockers with isosorbide mononitrate[63]. Regarding the primary prophylaxis, few studies have investigated the hemodynamic response to pharmacological therapy because of the difficulties in creating this kind of clinical trials.
In cases of acute variceal bleeding, the HVPG measurement was a good predictor of the prognosis and therapeutic efficacy of medication. Previous studies have suggested that an HVPG of > 12 mmHg is a good indicator of variceal bleeding in patients with LC[64,65]. In patients with acute variceal bleeding, the early prognosis in patients with alcoholic LC was closely related to the HVPG score measured within two days of hospital admission[66,67]. In addition, an HVPG of > 20 mmHg was significantly related to a long hospital stay, numerous blood transfusion, and a lower one-year mortality of 64%[49]. Albrades et al[66] demonstrated that an HVPG > 20 mmHg is independently related to the early prognosis of patients with acute variceal bleeding and should be treated with a vasoactive, antibiotic, or endoscopic therapy.
In patients with acute variceal bleeding, emergent endoscopic treatment [endoscopic injection sclerotherapy (EIS) or endoscopic band ligation (EBL)] has been commonly used. In one study, the HVPG was estimated to have significantly increased after endoscopic therapy compared to the pre-treatment HVPG score in the EBL (pre-treatment, 18 mmHg; post-treatment, 21 mmHg) and EIS groups (pre-treatment, 18 mmHg; post-treatment, 22 mmHg)[68]. However, in the EBL group, the HVPG recovered to the pretreatment values within two days after the endoscopic therapy, while in the EIS group, the HVPG score remained high during the five days of follow-up. As a result, the EIS was associated with a continued increase in HVPG in patients with acute variceal bleeding. It is well known that a reduction of 12 mmHg or more or at least than 20% from baseline HVPG score leads to decreased the risk of rebleeding and mortality[69,70]. Another report suggested that an HVPG reduction of > 10% from the baseline value is the best target to induce the greatest response to primary prophylaxis[71].
If patients with LC do not receive pharmacologic treatment, the risk of rebleeding increases to 55%-67%. The use of endoscopic therapy (EIS or EBL), a transjugular intrahepatic portosystemic shunt, or other types of shunts also reduce the risk of rebleeding[72,73]. However, the likelihood that a patient will fail to hemodynamically respond to treatment varies between from 45% to 63%[74]. Another study has suggested that HVPG monitoring was more effective when used with EBL and secondary prophylaxis therapy for variceal rebleeding than it was with EBL alone[75]. The HVPG has been shown to predict bleeding outcome such as variceal rebleeding and mortality[48,49,76].
Hepatocellular carcinoma (HCC) is the main cause of death in patients with LC[77]. A recent report proposed that non-selective beta-blockers decrease the incidence and progression of HCC via a reduction of the inflammatory materials from the gut to the liver and by inhibiting translocation[78]. Ripoll et al[79] suggested that portal hypertension is a significant predictor of HCC and an HVPG > 10 mmHg increase the risk of HCC by a six fold. In patients with decompensated alcoholic LC, the HVPG may be a predictor for the development of HCC[80]. Bruix et al[81] suggested that a high HVPG score is significantly related with decompensation after HCC operation. Another study demonstrated that a high HVPG score was related with mortality after liver resection for HCC[82]. However, in the field of HCC, few data are available about on role of HVPG. Further studies are needed.
Until now, the HVPG measurement has been used for the evaluation of LC prognosis in many studies[10]. The HVPG is a useful tool for the evaluation of viral recurrence after transplantation[83]. In viral LC, lamivudine monotherapy for chronic viral hepatitis has been found to reduce the HVPG effectively in patients with virologic suppression and biochemical remission[84]. One study demonstrated that the HVPG was positively associated with mortality and liver dysfunction after liver resection in patients with HCC[85]. They also concluded that preoperative HVPG measurements should be taken routinely for the evaluation of the prognosis. Moreover, Suk et al[86] demonstrated that a repeated HVPG measurement was necessary for the prediction of mortality in patients with decompensated LC. Measurement of the HVPG and albumin have been considered as significant predictors for the development of clinical decompensation in patients with compensated LC[87].
Suk et al[86] also reported that the HVPG measurement was better at predicting mortality than the model for end-stage liver disease (MELD) or MELD including serum sodium (MELD-Na) were. In patients with portal hypertension, monitoring the HVPG after the treatment provides substantial prognostic information[70]. However, other studies have suggested that the MELD-Na is the most predictive of one year mortality in patients with decompensated cirrhosis[88]. However, the combined use of the HVPG and the MELD/MELD-Na score does not improve the prognostic accuracy. To properly evaluate the prognostic accuracy of the MELD, MELD-Na, and HVPG, future studies are needed[86].
Recently, a new classification system for LC that combines histologic, clinical, hemodynamic and biologic features has been suggested (Table 2)[14,43,54]. This system classifies liver fibrosis according to the presence of compensation or decompensation which is mainly defined by clinical findings[44,89].
| Classification | Stages | ||||
| METAVIR score | F0-F3 | F4 | F4 | F4 | F4 |
| HVPG (mmHg) | > 6 | > 10 | > 12 | > 16 > 20 | |
| Clinical class | Stage 1 | Stage 2 | Stage 3 | Stage 4-5 | |
| Compensated | Compensated | Decompensated | Decompensated | ||
| Varices | Varices Ascites | Variceal bleeding Ascites Other complications | |||
| 1-yr mortality (%) | 1 | 3 | 10-30 | 60-100 | |
At the METAVIR F1-F3 stages (the non-cirrhosis stage of chronic liver disease) without histologic or clinical evidence of LC, the HVPG is expected to be within the normal range (1-5 mmHg). The cirrhotic stage of METAVIR F4 is sub-classified into two stages: compensation and decompensation. Clinical decompensation is defined as the development of ascites, a variceal hemorrhage, encephalopathy, and/or jaundice. The compensated stage can be further classified into stage 1 without varices or stage 2 with varices[54]. Portal hypertension is considered moderate or subclinical when 6 mmHg < HVPG ≤ 10 mmHg (stage 1 compensated LC)[54]. A clinically significant case is defined when the HVPG is > 10 mmHg (stage 2 compensated LC). A severe case is defined when the HVPG is > 12 mmHg (stage 3 or 4 decompensated LC) (Table 2).
In a previous study, the one year outcome probabilities were calculated according to the clinical stage of LC[44]. Recently, D’Amico et al[90] proposed that the development of varices and decompensating events in cirrhosis should be divided into five prognostic stages with significantly increasing mortality risks. However, no definite staging system is yet widely accepted for clinical practice in chronic liver disease and there is little evidence available regarding the correlation between hemodynamic (exact score according to stages), pathologic (Laennec fibrosis scoring system according to stages), and clinical staging (complications of LC according to stages) of chronic liver disease[91-94]. Therefore, further studies on the recent classification system that combines histologic, clinical, hemodynamic and biologic findings are needed in the future.
The HVPG measurement is a safe, simple, and reproducible method of quantifying liver fibrosis. The recent concept considers LC as a dynamic and potentially reversible disease. There are many stages in liver fibrosis of chronic liver disease. Of these stages, HVPG measurement is a method of evaluating the presence and severity of liver fibrosis. The HVPG measurement has been used clinically for fibrosis diagnosis, risk stratification, preoperative screening for liver resection, monitoring the efficacy of medical treatments, and assessing the prognosis of liver fibrosis. HVPG measurement, along with monitoring stage the liver fibrosis, will play important roles in the field of chronic liver disease. Therefore, measuring HVPG in addition to monitoring hemodynamic effects or staging liver fibrosis will play an important role in the management of chronic liver disease.
P- Reviewer: Hori T, Villa-Trevino S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Karsdal MA, Krarup H, Sand JM, Christensen PB, Gerstoft J, Leeming DJ, Weis N, Schaffalitzky de Muckadell OB, Krag A. Review article: the efficacy of biomarkers in chronic fibroproliferative diseases - early diagnosis and prognosis, with liver fibrosis as an exemplar. Aliment Pharmacol Ther. 2014;40:233-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1019] [Cited by in F6Publishing: 1075] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 3. | Germani G, Hytiroglou P, Fotiadu A, Burroughs AK, Dhillon AP. Assessment of fibrosis and cirrhosis in liver biopsies: an update. Semin Liver Dis. 2011;31:82-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 79] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 4. | Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069-1075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1066] [Cited by in F6Publishing: 1113] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 5. | Brenner DA. Reversibility of liver fibrosis. Gastroenterol Hepatol (N Y). 2013;9:737-739. [PubMed] [Cited in This Article: ] |
| 6. | Sanyal AJ, Bosch J, Blei A, Arroyo V. Portal hypertension and its complications. Gastroenterology. 2008;134:1715-1728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 223] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 7. | de Franchis R, Primignani M. Natural history of portal hypertension in patients with cirrhosis. Clin Liver Dis. 2001;5:645-663. [PubMed] [Cited in This Article: ] |
| 8. | Burroughs AK, McCormick PA. Natural history and prognosis of variceal bleeding. Baillieres Clin Gastroenterol. 1992;6:437-450. [PubMed] [Cited in This Article: ] |
| 9. | Krook H. Estimation of portal venous pressure by occlusive hepatic vein catheterization. Scand J Clin Lab Invest. 1953;5:285-292. [PubMed] [Cited in This Article: ] |
| 10. | Armonis A, Patch D, Burroughs A. Hepatic venous pressure measurement: an old test as a new prognostic marker in cirrhosis? Hepatology. 1997;25:245-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Burroughs AK, Thalheimer U. Hepatic venous pressure gradient in 2010: optimal measurement is key. Hepatology. 2010;51:1894-1896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Kumar A, Sharma P, Sarin SK. Hepatic venous pressure gradient measurement: time to learn! Indian J Gastroenterol. 2008;27:74-80. [PubMed] [Cited in This Article: ] |
| 13. | Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 451] [Cited by in F6Publishing: 454] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 14. | Suk KT. Hepatic venous pressure gradient: clinical use in chronic liver disease. Clin Mol Hepatol. 2014;20:6-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | Merkel C, Montagnese S. Hepatic venous pressure gradient measurement in clinical hepatology. Dig Liver Dis. 2011;43:762-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Hong WK, Kim MY, Baik SK, Shin SY, Kim JM, Kang YS, Lim YL, Kim YJ, Cho YZ, Hwang HW. The usefulness of non-invasive liver stiffness measurements in predicting clinically significant portal hypertension in cirrhotic patients: Korean data. Clin Mol Hepatol. 2013;19:370-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Suk KT, Kim DY, Sohn KM, Kim DJ. Biomarkers of liver fibrosis. Adv Clin Chem. 2013;62:33-122. [PubMed] [Cited in This Article: ] |
| 18. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1928] [Cited by in F6Publishing: 2053] [Article Influence: 128.3] [Reference Citation Analysis (0)] |
| 19. | Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology. 2004;39:273-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 386] [Cited by in F6Publishing: 378] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 20. | Brenner DA. Molecular pathogenesis of liver fibrosis. Trans Am Clin Climatol Assoc. 2009;120:361-368. [PubMed] [Cited in This Article: ] |
| 21. | Wells RG, Kruglov E, Dranoff JA. Autocrine release of TGF-beta by portal fibroblasts regulates cell growth. FEBS Lett. 2004;559:107-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Bachem MG, Melchior R, Gressner AM. The role of thrombocytes in liver fibrogenesis: effects of platelet lysate and thrombocyte-derived growth factors on the mitogenic activity and glycosaminoglycan synthesis of cultured rat liver fat storing cells. J Clin Chem Clin Biochem. 1989;27:555-565. [PubMed] [Cited in This Article: ] |
| 23. | Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 586] [Cited by in F6Publishing: 576] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 24. | Friedman SL. Hepatic fibrosis -- overview. Toxicology. 2008;254:120-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 258] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 25. | Melton AC, Datta A, Yee HF. [Ca2+]i-independent contractile force generation by rat hepatic stellate cells in response to endothelin-1. Am J Physiol Gastrointest Liver Physiol. 2006;290:G7-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Han YP. Matrix metalloproteinases, the pros and cons, in liver fibrosis. J Gastroenterol Hepatol. 2006;21 Suppl 3:S88-S91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Niiya M, Uemura M, Zheng XW, Pollak ES, Dockal M, Scheiflinger F, Wells RG, Zheng XL. Increased ADAMTS-13 proteolytic activity in rat hepatic stellate cells upon activation in vitro and in vivo. J Thromb Haemost. 2006;4:1063-1070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Murphy FR, Issa R, Zhou X, Ratnarajah S, Nagase H, Arthur MJ, Benyon C, Iredale JP. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J Biol Chem. 2002;277:11069-11076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 350] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 29. | Wanless IR, Wong F, Blendis LM, Greig P, Heathcote EJ, Levy G. Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension. Hepatology. 1995;21:1238-1247. [PubMed] [Cited in This Article: ] |
| 30. | Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1446] [Cited by in F6Publishing: 1428] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 31. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1139] [Cited by in F6Publishing: 1188] [Article Influence: 118.8] [Reference Citation Analysis (0)] |
| 32. | Schaffner F, Poper H. Capillarization of hepatic sinusoids in man. Gastroenterology. 1963;44:239-242. [PubMed] [Cited in This Article: ] |
| 33. | Desmet VJ, Roskams T. Cirrhosis reversal: a duel between dogma and myth. J Hepatol. 2004;40:860-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | Menghini G. One-second needle biopsy of the liver. Gastroenterology. 1958;35:190-199. [PubMed] [Cited in This Article: ] |
| 35. | Asselah T, Marcellin P, Bedossa P. Improving performance of liver biopsy in fibrosis assessment. J Hepatol. 2014;61:193-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Hytiroglou P, Snover DC, Alves V, Balabaud C, Bhathal PS, Bioulac-Sage P, Crawford JM, Dhillon AP, Ferrell L, Guido M. Beyond “cirrhosis”: a proposal from the International Liver Pathology Study Group. Am J Clin Pathol. 2012;137:5-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Schmeltzer PA, Talwalkar JA. Noninvasive tools to assess hepatic fibrosis: ready for prime time? Gastroenterol Clin North Am. 2011;40:507-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1193] [Cited by in F6Publishing: 1334] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 39. | Manning DS, Afdhal NH. Diagnosis and quantitation of fibrosis. Gastroenterology. 2008;134:1670-1681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 321] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 40. | Hall AR, Tsochatzis E, Morris R, Burroughs AK, Dhillon AP. Sample size requirement for digital image analysis of collagen proportionate area in cirrhotic livers. Histopathology. 2013;62:421-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Bosch J, Garcia-Pagán JC, Berzigotti A, Abraldes JG. Measurement of portal pressure and its role in the management of chronic liver disease. Semin Liver Dis. 2006;26:348-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 153] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 42. | Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology. 2004;39:280-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 406] [Cited by in F6Publishing: 363] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 43. | Albilllos A, Garcia-Tsao G. Classification of cirrhosis: the clinical use of HVPG measurements. Dis Markers. 2011;31:121-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 20] [Reference Citation Analysis (0)] |
| 44. | D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1892] [Cited by in F6Publishing: 1894] [Article Influence: 105.2] [Reference Citation Analysis (1)] |
| 45. | Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254-2261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 714] [Cited by in F6Publishing: 612] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 46. | Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R, Patch D. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 747] [Cited by in F6Publishing: 703] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 47. | Gluud C, Henriksen JH, Nielsen G. Prognostic indicators in alcoholic cirrhotic men. Hepatology. 1988;8:222-227. [PubMed] [Cited in This Article: ] |
| 48. | Merkel C, Bolognesi M, Bellon S, Zuin R, Noventa F, Finucci G, Sacerdoti D, Angeli P, Gatta A. Prognostic usefulness of hepatic vein catheterization in patients with cirrhosis and esophageal varices. Gastroenterology. 1992;102:973-979. [PubMed] [Cited in This Article: ] |
| 49. | Moitinho E, Escorsell A, Bandi JC, Salmerón JM, García-Pagán JC, Rodés J, Bosch J. Prognostic value of early measurements of portal pressure in acute variceal bleeding. Gastroenterology. 1999;117:626-631. [PubMed] [Cited in This Article: ] |
| 50. | Suk KT, Kim HC, Namkung S, Han SH, Choi KC, Park SH, Sung HT, Kim CH, Kim SH, Ham YL. Diagnostic accuracy of hepatic venous pressure gradient measurement in the prediction of stage 1 compensated liver cirrhosis in patients with chronic hepatitis B. Eur J Gastroenterol Hepatol. 2013;25:1170-1176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Kumar M, Kumar A, Hissar S, Jain P, Rastogi A, Kumar D, Sakhuja P, Sarin SK. Hepatic venous pressure gradient as a predictor of fibrosis in chronic liver disease because of hepatitis B virus. Liver Int. 2008;28:690-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Suk KT, Kim DJ, Kim CH, Park SH, Cheong JY, Cho SW, Choi JY, Han KH, Sung HT, Hong SH. Diagnostic accuracy of biomarkers measured in the hepatic vein and peripheral vein in the prediction of advanced fibrosis in patients with chronic viral hepatitis. Clin Biochem. 2012;45:1075-1080. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 53. | Ripoll C. Hepatic venous pressure gradient and outcomes in cirrhosis. J Clin Gastroenterol. 2007;41 Suppl 3:S330-S335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 54. | Garcia-Tsao G, Friedman S, Iredale J, Pinzani M. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445-1449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 361] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 55. | Castera L, Pinzani M, Bosch J. Non invasive evaluation of portal hypertension using transient elastography. J Hepatol. 2012;56:696-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 220] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 56. | Vizzutti F, Arena U, Romanelli RG, Rega L, Foschi M, Colagrande S, Petrarca A, Moscarella S, Belli G, Zignego AL. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007;45:1290-1297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 527] [Cited by in F6Publishing: 493] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 57. | Augustin S, Millán L, González A, Martell M, Gelabert A, Segarra A, Serres X, Esteban R, Genescà J. Detection of early portal hypertension with routine data and liver stiffness in patients with asymptomatic liver disease: a prospective study. J Hepatol. 2014;60:561-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 58. | Colli A, Fraquelli M, Casazza G, Conte D, Nikolova D, Duca P, Thorlund K, Gluud C. The architecture of diagnostic research: from bench to bedside--research guidelines using liver stiffness as an example. Hepatology. 2014;60:408-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 59. | Bureau C, Metivier S, Peron JM, Selves J, Robic MA, Gourraud PA, Rouquet O, Dupuis E, Alric L, Vinel JP. Transient elastography accurately predicts presence of significant portal hypertension in patients with chronic liver disease. Aliment Pharmacol Ther. 2008;27:1261-1268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 260] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 60. | Carrión JA, Navasa M, Bosch J, Bruguera M, Gilabert R, Forns X. Transient elastography for diagnosis of advanced fibrosis and portal hypertension in patients with hepatitis C recurrence after liver transplantation. Liver Transpl. 2006;12:1791-1798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 300] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 61. | D’Amico G, Luca A. Natural history. Clinical-haemodynamic correlations. Prediction of the risk of bleeding. Baillieres Clin Gastroenterol. 1997;11:243-256. [PubMed] [Cited in This Article: ] |
| 62. | Kim JN, Sohn KM, Kim MY, Suk KT, Jeong SW, Jung HE, Lee SH, Kim SG, Jang JY, Kim YS. Relationship between the hepatic venous pressure gradient and first variceal hemorrhage in patients with cirrhosis: a multicenter retrospective study in Korea. Clin Mol Hepatol. 2012;18:391-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Merkel C, Bolognesi M, Sacerdoti D, Bombonato G, Bellini B, Bighin R, Gatta A. The hemodynamic response to medical treatment of portal hypertension as a predictor of clinical effectiveness in the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology. 2000;32:930-934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 182] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 64. | Vinel JP, Cassigneul J, Levade M, Voigt JJ, Pascal JP. Assessment of short-term prognosis after variceal bleeding in patients with alcoholic cirrhosis by early measurement of portohepatic gradient. Hepatology. 1986;6:116-117. [PubMed] [Cited in This Article: ] |
| 65. | Garcia-Tsao G, Groszmann RJ, Fisher RL, Conn HO, Atterbury CE, Glickman M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology. 1985;5:419-424. [PubMed] [Cited in This Article: ] |
| 66. | Abraldes JG, Villanueva C, Bañares R, Aracil C, Catalina MV, Garci A-Pagán JC, Bosch J. Hepatic venous pressure gradient and prognosis in patients with acute variceal bleeding treated with pharmacologic and endoscopic therapy. J Hepatol. 2008;48:229-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 195] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 67. | Vlachogiannakos J, Kougioumtzian A, Triantos C, Viazis N, Sgouros S, Manolakopoulos S, Saveriadis A, Markoglou C, Economopoulos T, Karamanolis DG. Clinical trial: The effect of somatostatin vs. octreotide in preventing post-endoscopic increase in hepatic venous pressure gradient in cirrhotics with bleeding varices. Aliment Pharmacol Ther. 2007;26:1479-1487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 68. | Monescillo A, Martínez-Lagares F, Ruiz-del-Arbol L, Sierra A, Guevara C, Jiménez E, Marrero JM, Buceta E, Sánchez J, Castellot A. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology. 2004;40:793-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 353] [Cited by in F6Publishing: 305] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 69. | Albillos A, Bañares R, González M, Ripoll C, Gonzalez R, Catalina MV, Molinero LM. Value of the hepatic venous pressure gradient to monitor drug therapy for portal hypertension: a meta-analysis. Am J Gastroenterol. 2007;102:1116-1126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 70. | D’Amico G, Garcia-Pagan JC, Luca A, Bosch J. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology. 2006;131:1611-1624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 326] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 71. | Villanueva C, Aracil C, Colomo A, Hernández-Gea V, López-Balaguer JM, Alvarez-Urturi C, Torras X, Balanzó J, Guarner C. Acute hemodynamic response to beta-blockers and prediction of long-term outcome in primary prophylaxis of variceal bleeding. Gastroenterology. 2009;137:119-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 160] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 72. | Bosch J, García-Pagán JC. Prevention of variceal rebleeding. Lancet. 2003;361:952-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 267] [Article Influence: 12.7] [Reference Citation Analysis (2)] |
| 73. | D’Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis. 1999;19:475-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 583] [Cited by in F6Publishing: 592] [Article Influence: 24.7] [Reference Citation Analysis (1)] |
| 74. | Sharara AI, Rockey DC. Gastroesophageal variceal hemorrhage. N Engl J Med. 2001;345:669-681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 298] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 75. | Targownik LE, Spiegel BM, Dulai GS, Karsan HA, Gralnek IM. The cost-effectiveness of hepatic venous pressure gradient monitoring in the prevention of recurrent variceal hemorrhage. Am J Gastroenterol. 2004;99:1306-1315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 76. | Patch D, Armonis A, Sabin C, Christopoulou K, Greenslade L, McCormick A, Dick R, Burroughs AK. Single portal pressure measurement predicts survival in cirrhotic patients with recent bleeding. Gut. 1999;44:264-269. [PubMed] [Cited in This Article: ] |
| 77. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3249] [Cited by in F6Publishing: 3477] [Article Influence: 289.8] [Reference Citation Analysis (3)] |
| 78. | Thiele M, Wiest R, Gluud LL, Albillos A, Krag A. Can non-selective beta-blockers prevent hepatocellular carcinoma in patients with cirrhosis? Med Hypotheses. 2013;81:871-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Ripoll C, Groszmann RJ, Garcia-Tsao G, Bosch J, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol. 2009;50:923-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 264] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 80. | Kim MY, Baik SK, Yea CJ, Lee IY, Kim HJ, Park KW, Kim HK, Suk KT, Kim JW, Kim HS. Hepatic venous pressure gradient can predict the development of hepatocellular carcinoma and hyponatremia in decompensated alcoholic cirrhosis. Eur J Gastroenterol Hepatol. 2009;21:1241-1246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 81. | Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, Visa J, Bru C, Rodés J. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018-1022. [PubMed] [Cited in This Article: ] |
| 82. | Hidaka M, Takatsuki M, Soyama A, Tanaka T, Muraoka I, Hara T, Kuroki T, Kanematsu T, Eguchi S. Intraoperative portal venous pressure and long-term outcome after curative resection for hepatocellular carcinoma. Br J Surg. 2012;99:1284-1289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 83. | Blasco A, Forns X, Carrión JA, García-Pagán JC, Gilabert R, Rimola A, Miquel R, Bruguera M, García-Valdecasas JC, Bosch J. Hepatic venous pressure gradient identifies patients at risk of severe hepatitis C recurrence after liver transplantation. Hepatology. 2006;43:492-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 240] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 84. | Manolakopoulos S, Triantos C, Theodoropoulos J, Vlachogiannakos J, Kougioumtzan A, Papatheodoridis G, Tzourmakliotis D, Karamanolis D, Burroughs AK, Archimandritis A. Antiviral therapy reduces portal pressure in patients with cirrhosis due to HBeAg-negative chronic hepatitis B and significant portal hypertension. J Hepatol. 2009;51:468-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 85. | Boleslawski E, Petrovai G, Truant S, Dharancy S, Duhamel A, Salleron J, Deltenre P, Lebuffe G, Mathurin P, Pruvot FR. Hepatic venous pressure gradient in the assessment of portal hypertension before liver resection in patients with cirrhosis. Br J Surg. 2012;99:855-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 86. | Suk KT, Kim CH, Park SH, Sung HT, Choi JY, Han KH, Hong SH, Kim DY, Yoon JH, Kim YS. Comparison of hepatic venous pressure gradient and two models of end-stage liver disease for predicting the survival in patients with decompensated liver cirrhosis. J Clin Gastroenterol. 2012;46:880-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 87. | Rincón D, Lo Iacono O, Tejedor M, Hernando A, Ripoll C, Catalina MV, Salcedo M, Matilla A, Senosiain M, Clemente G. Prognostic value of hepatic venous pressure gradient in patients with compensated chronic hepatitis C-related cirrhosis. Scand J Gastroenterol. 2013;48:487-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 88. | Park SH, Park HY, Kang JW, Park JS, Shin KJ, Kim CH, Suk KT, Baik GH, Kim JB, Kim DJ. Identification of patients with decompensated cirrhosis at high risk for death: improving the prediction by hepatic venous pressure gradient? Hepatogastroenterology. 2012;59:2548-2551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 89. | Castera L, Pinzani M. Biopsy and non-invasive methods for the diagnosis of liver fibrosis: does it take two to tango? Gut. 2010;59:861-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 90. | D’Amico G, Pasta L, Morabito A, D’Amico M, Caltagirone M, Malizia G, Tinè F, Giannuoli G, Traina M, Vizzini G. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther. 2014;39:1180-1193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 305] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 91. | Qi X, Zhou F, Lv H, Chen H, Xu W, Xing S, Wang F, Yang C. A novel noninvasive assessment of hepatic venous pressure gradient and portal pressure computed from computed tomography angiography. Arch Med Sci. 2014;10:1052-1054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 92. | Qi XS, Fan DM. Hepatic venous pressure gradient measurement before TIPS for acute variceal bleeding. World J Gastroenterol. 2014;20:7523-7524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 93. | Huang SY, Abdelsalam ME, Harmoush S, Ensor JE, Chetta JA, Hwang KP, Stafford RJ, Madoff DC, Avritscher R. Evaluation of liver fibrosis and hepatic venous pressure gradient with MR elastography in a novel swine model of cirrhosis. J Magn Reson Imaging. 2014;39:590-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 94. | Woolfson J, John P, Kamath B, Ng VL, Ling SC. Measurement of hepatic venous pressure gradient is feasible and safe in children. J Pediatr Gastroenterol Nutr. 2013;57:634-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |